Halotron BrX® Clean Agent
High Performance Clean Agent that is designed for applications where efficient firefighting performance is the most important factor
Clean Agent
Light Weight
Extended Application Distance
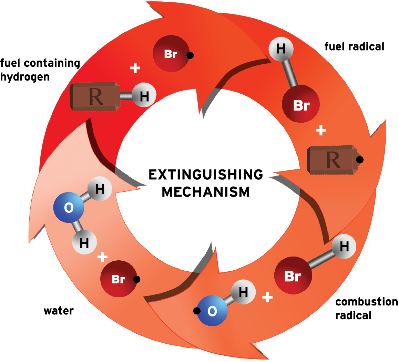

How Does It Work?
Bromine (Br) from the Halotron BrX® clean agent scavenges the chemical reaction by stripping hydrogen from the fuel source, creating HBr, which interrupts the combustion cycle by reacting with combustion radicals to create water. The bromine is then free to start the process again. This chain breaking scavenging along with physical properties of the agent that cool the fire result in a quick extinguishment of the fire.


Extraordinarily Low Global Warming Potential
For its chemical class, Halotron BrX® has an extraordinarily low global warming potential (less than 0.50, where CO2 = 1.0, 100 yr integrated time horizon). This is in contrast to some other conventional clean agents that have global warming potentials above 3,000. The Ozone Depletion Potential is near zero (0.0028, where CFC-11=1.0). The atmospheric lifetime is a very short 7 days. This property contributes to its favorable minimal environmental impact, which has resulted in this material not being subject to ozone depletion or global warming regulations.

